 TESTAEDGE Cream for Men Testosterone Booster Anti-aging Ed Libido 4 Oz for sale online | eBay
TESTAEDGE Cream for Men Testosterone Booster Anti-aging Ed Libido 4 Oz for sale online | eBayTop Menu Browse Products Empower offers an expansive composite form in several treatment areas that address what is important for both prescriptors and patients: cost, comfort, compliance and quality confidence. CompanyEmpower is a leading company in the pharmacy industry and subcontracting with a record of 11 years of excellence and innovation in the pharmaceutical compound. Learn more about how we deliver value to our patients and prescribers. Top Menu Browse Products Empower offers an expansive composite form in several treatment areas that address what is important for both prescriptors and patients: cost, comfort, compliance and quality confidence. CompanyEmpower is a leading company in the pharmacy industry and subcontracting with a record of 11 years of excellence and innovation in the pharmaceutical compound. Learn more about how we deliver value to our patients and prescribers. Testosterone creamOverview of Testosterone CreamDosage Strengths of Testosterone Cream1 - 200 mg/mL 30 mL Topi-Click DispenserGeneral InformationTestosterone is the primary androgen found in the body. Endogenous testosterone is synthesized by cells in the testis, ovary and adrenal cortex. Therapetically, testosterone is used in the management of hypogonadism, either congenital or acquired. Testosterone is also the most effective exogenous androgen for palliative treatment of breast carcinoma in postmenopausal women. Testosterone was used in 1938 and approved by the FDA in 1939. Anabolic steroids, derived from testosterone, have been used illicitly and are now controlled substances. Testosterone, like many anabolic steroids, was classified as a controlled substance in 1991. Developed in the United States by Uniumed Pharmaceuticals such as AndroGel, the testosterone cream was approved in 2000 for the treatment of testosterone deficiency, which often results in a variety of hypogonal conditions of humor and energy to sexual dysfunctions, as well as a treatment for several conditions related to injuries such as those experienced by serious victims of burns and accidents. A very popular form of testosterone, AndroGel is sold worldwide under a couple of less popular brand names and trade more remarkable Testogel (manufactured in the UK by Laboratoires Besins and distributed by Bayer), Testim (manufactured in the U.S. by Auxilium Pharmaceuticals, Inc.), and several generic versions often sold under the name creams. The transdermal delivery system of testosterone cream is directed to the same, or at least to very similar body regions such as injections and other forms of testosterone. More specifically, the maximum absorption of testosterone cream is achieved when (such as testosterone injectable) is given to densely muscle body regions. Since the higher amounts of muscle at the application point amount to a higher number of testosterone absorbing capillaries, testosterone can be more quickly transferred to the bloodstream. Mechanism of ActionEndogenous testosterone is responsible for sexual maturation in all stages of development throughout life. Synthetically, it is prepared from cholesterol. The function of androgens in male development begins in the fetus, is crucial during puberty, and continues to play an important role in the adult male. Women also secrete small amounts of ovarian testosterone. The secretion of androgens from the adrenal cortex is insufficient to maintain male sexuality. Increased androgen plasma concentrations suppress the gonadotropin-reducing hormone (reduction of endogenous testosterone), luteinizing hormone and follicle-stimulating hormone by a negative feedback mechanism. Testosterone also affects the formation of erythropoietin, the balance of calcium and glucose in the blood. Androgens have a high lipid solubility, allowing them to quickly enter white tissue cells. Within the cells, testosterone experiences enzyme conversion to 5-alpha-dihydrotestosterone and forms a loose complex with cystolic receptors. Androgen action arises from transcription initiation and cell changes in the core produced by this steroid receptor complex. Normally, endogenous androgens stimulate RNA polymerase, leading to increased protein production. These proteins are responsible for normal male sexual development, including prostate growth and maturation, seminal vesicle, penis and scrotum. During puberty, androgens cause a sudden increase in muscle growth and development, with the redistribution of body fat. The changes also take place in the larynx and vocal cords, deepening the voice. Puberty is complete with beard development and body hair growth. The fusion of epiphytes and the termination of growth is also governed by androgens, such as the maintenance of spermatogenesis. When endogenous androgens are not available, the use of exogenous androgens is necessary for normal male growth and development. PharmacokineticsStesterone is administered intramuscularly (as primary); by subcutaneous injection; to skin as a topical gel, solution, ointment or transdermal systems for transdermal absorption; by implanting protracted pellets, or by means of oral systems. In serum, testosterone is linked to protein. It has a high affinity for sexual hormone binding globulin (SHBG) and a low affinity for albumin. The portion of discontin-bound is freely dissociated. Affinity for SHBG changes throughout life. It is high during prepubertry, it decreases during adolescence and adult life, then it rises again in old age. The active metabolite DHT has a greater affinity for SHBG than testosterone. The half life of elimination is 10 to 100 minutes and depends on the amount of free testosterone in the plasma. Testosterone is primarily metabolized in the liver to several 17-keto steroids. It is a substrate for the P450 hepatic cytochrome (CYP) 3A4 isoenzyme. Estradiol and dihydrotestosterone (DHT) are the main active metabolites, and DHT suffers more metabolism. Testosterone activity seems to depend on the formation of DHT, which binds to cytosol receptor proteins. Additional DHT metabolism takes place in reproductive tissues. About 90% of an intramuscular dose of testosterone is excreted in the urine as glucuronic and sulfuric acid conjugates. About 6% are excreted in the feces, largely unconjugated. There is considerable variation in the average life of testosterone as indicated in literature, which goes from 10 to 100 minutes. Citochrome Affected P450 isoenzymes and drug carriers: CYP3A4, P-gpTestosterone is a substrate for CYP3A4 and is also transported by and a P-glycoprotein inhibitor (P-gp) transport. Pharmacokinetics of the route: Topic route: About 10% of a applied topic dose of testosterone skin gel or ointment is absorbed systemically with a daily dose; the absorption of the gel and the solution of the skin occurs continuously during the 24-hour dosing interval, which indicates that the skin acts as a reservoir for sustained release. The application of testosterone or gel solution offers circulating physiological testosterone that resembles the normal concentration range seen in healthy men. Stage-state concentrations are achieved after approximately 14 days of solution application; when the solution is stopped, pretreatment testosterone concentrations are achieved in approximately 7 to 10 days. IndicationsTestosterone is mainly used in men who do not make enough testosterone naturally (hypogonadism), as well as in specific cases of teenagers to induce puberty in those with delayed puberty. Contraindications/Precautions Your health care provider needs to know if you have any of these conditions: breast cancer; breathing problems while sleeping; diabetes; heart disease; if a female partner is pregnant or is trying to become pregnant; kidney disease; liver disease; lung disease; prostate cancer, enlargement; any unusual or allergic reaction to testosterone or other products; pregnant or trying to become pregnant; breastfeeding. Your healthcare provider will need to have a regular blood test while you are in testosterone. This medication is prohibited from being used in athletes by most athletic organizations. The gels and topical solutions are usually flammable, so you should avoid exposure to fire, flame and smoking using any topical gel or testosterone solution formulation. Testosterone can stimulate the growth of cancerous tissue and is contraindicated in male patients with prostate cancer or breast cancer. Patients with prostatic hypertrophy should be treated with caution because androgen therapy may cause a worsening of signs and symptoms of benign prostatic hypertrophy and may increase the risk of developing malignancy. Older patients and other patients with clinical or demographic characteristics that are recognized as associated with a higher risk of prostate cancer should be evaluated for the presence of prostate cancer before starting testosterone replacement therapy. In patients receiving testosterone therapy, prostate cancer surveillance should be consistent with current practices for eugonadal men. The replacement of testosterone is not indicated in geriatric patients who have age-related hypogonadism only or andropause because there is not enough safety and efficacy information to support such use. In addition, the long-term effectiveness and security of the testosterone topical solution in patients over 65 years of age has not been determined because of an insufficient number of geriatric patients involved in controlled trials. According to the Cervece Criteria, testosterone is considered a potentially inappropriate medication (PIM) for use in geriatric patients and should be avoided due to the potential of heart problems and their contraindication in prostate cancer. The panel of beer experts considers that the use of moderate to severe hypogonadism is acceptable. Due to the reduction of drug clearance and increased risk of drug build-up, patients with liver disease or liver dysfunction should be prescribed with caution. In addition, the secondary edema to water and sodium retention may occur during androgen treatment. Use testosterone with caution in patients with liver disease; kidney disease, including nephritis and nephrosis; pre-existing edema; or heart disease, including heart failure, coronary disease, and myocardial infarction (MI), as fluid retention may aggravate these conditions. In addition, the possible association between the use of testosterone and the increased risk of serious cardiovascular events, regardless of pre-existing heart disease, is being investigated. An observational study in the U.S. Veterans' Health System included adult male patients of an average age of 60 years. Patients (n = 8709) suffer coronary angiography with a low serum testosterone concentration of = 65 years old) adult males (n = 55.593). The incidence rate of MI that occurred within 90 days after the initial testosterone prescription was compared to the rate of MI incidence that occurred in the year prior to the first prescription. Among older men, an increase of 2 times in the risk of MI was observed within the 90-day window; among younger men with pre-existing history of heart disease, an increase of 2 to 3 times of risk of MI was observed. However, there was no greater risk in younger men without a history of heart disease. In light of these findings, the FDA announced in early 2014 a review of the possible link between testosterone therapy and serious cardiovascular events. FDA has not concluded that the treatment of testosterone approved by FDA increases the risk of stroke, MI or death. However, health professionals are urged to consider carefully whether treatment benefits are likely to exceed potential risks. The FDA will communicate its final conclusions and recommendations when the evaluation is complete. Treatment of hypogonadal men with testosterone esters can enhance sleep apnea, especially in patients who have risk factors for apnea such as obesity or chronic lung disease. In addition, the safety and effectiveness of the topical testosterone and intranasal gel solution has not been established in obese males with BMI Ø 35 kg/m2. Patients who receive high doses of testosterone are at risk of polycytemia. Patients receiving testosterone should have their hemoglobin and hematocrite levels measured to detect polycytemia. Testosterone is contraindicated during pregnancy due to the likely adverse effects on the fetus (the X category of AFD pregnancy risk). Women with child potential who receive testosterone treatment should use appropriate contraceptive methods. Because testosterone is not used during pregnancy, there should be no particular reason for administering products to women during childbirth or obstetric delivery; safety and effectiveness in these environments have not been established. Testosterone is specifically contraindicated in women; the medication is only for men; the dose form provides testosterone in excess of what should be prescribed to women under certain endocrine situations. In addition, the products of the brand Androgel, Androderm, Aveed, Fortesta and Striant are not indicated for use in women due to the lack of controlled evaluations and/or the potential for virilizing effects. Patients who receive other forms of testosterone therapy should be closely monitored to detect signs of virilization (deepification of voice, hirsutism, acne, clitoris and menstrual irregularities). In high doses, virilization is common and is not avoided by concomitant use of estrogens. It can be judged that some virilization is acceptable during the treatment of breast carcinoma; however, if mild virilism is evident, it is necessary that drug therapy be interrupted to prevent long-term virilization. Females should be aware that accidental exposure to some forms of testosterone doses (i.e. ointments, solutions and gels) may occur if they come into direct contact with a treated patient. In clinical studies, within 2 to 12 hours of gel application by male subjects, 15 minutes of vigorous skin contact sessions with a female couple resulted in levels of serum female testosterone. When the clothes covered the site treated in the male, the transfer of testosterone to the female was prevented. Accidental exposure to the topic testosterone gel has also occurred in pediatric patients after contact between the child and the application site in treated individuals. The reported adverse events include genital expansion, pubic hair development, advanced bone age, increased libido and aggressive behavior. Symptoms were resolved in most patients when exposure to the product stopped. However, in some patients, the enlargement of the genitals and the advanced bone age did not completely return to the expected measurements. The FDA recommends taking precautions to minimize the potential for accidental exposure of topical testosterone products by washing hands with warm water and soap after each application, covering the application site with clothing, and eliminating soap and water medications when contact with another person is anticipated. In the case of direct contact from skin to skin with the testosterone application site, the untreated person should wash the area with soap and water as soon as possible. The topical solution of testosterone, transdermal patches and gels are contraindicated in breastfeeding women. It is recommended to avoid other testosterone formulations during breastfeeding as well. The distribution of testosterone to breast milk has not been determined; it is unclear whether the exposure would increase above the levels normally found in human milk. Significant exposure to this androgen through breastfeeding may have adverse androgenic effects on the baby and the medication may also interfere with the proper establishment of breastfeeding in the mother. Historically, testosterone/androgens have been used adjunctively for the suppression of breastfeeding. Alternative methods for breastfeeding are recommended for infants who receive testosterone therapy. Androgen therapy, such as testosterone, can result in diabetic loss of control and should be used with caution in patients with diabetes mellitus. A close follow-up of blood glucose is recommended. Testosterone has induced osteolysis and should be used with caution in patients with hypercalcemia, which can be exacerbated in patients with metastatic breast cancer. The administration of the undecanate of testosterone has been associated with cases of severe reactions of pulmonary oil microembolism (POME) as well as anaphylactoid reactions. Reported cases of POME reactions occurred during or immediately after an intramuscular injection of 1000 mg of undecanate testosterone. Symptoms included: cough, coughing, dyspnea, hyperhydrosis, tightening of the throat, chest pain, dizziness and syncope. Most of the cases lasted a few minutes and were resolved with support measures; however, some lasted for several hours, and some of them required emergency care and/or hospitalization. When administering undeanatecanate testosterone, doctors should take care of deeply injecting into the gluteal muscle, avoiding intravascular injection. In addition to POME reactions, episodes of anaphylaxis have been reported, including potentially fatal reactions, following intramuscular injection of a testosterone deanate. Patients with suspicious hypersensitivity reactions should not be removed with undecanate testosterone. After each administration, monitor the patient for 30 minutes and provide appropriate medical treatment in case of severe reactions of POME or anaphylactoid. Due to the risk of severe reactions of POME and anaphylaxia, undecanate testosterone (Aveed) is only available through a restricted program called REMS Aveed Program. Clinicians who wish to prescribe Aveed must be certified with the REMS Program to order or dispens the product. Medical care settings should also be certified with the REMS Program and should have the resources to provide emergency medical treatment in cases of severe PME and anaphylaxia. More information is available at www. AveedREMS.com or call 1-855-755-0494. Intranasal formulations of testosterone (e.g., Natesto) are not recommended for individuals with a history of nasal disorders such as nasal polyps; nasal septal perforation; nasal surgery; nasal trauma resulting from nasal fracture in the previous 6 months or nasal fracture that caused a previously diverted nasal septus; sinusal surgery or sinusal disease. In addition, the safety and effectiveness of intranasal testosterone has not been evaluated in individuals with mucous inflammatory disorders such as Sjogren syndrome. Patients with rhinorene (rhinitis) receiving intranasal testosterone formulations may experience decreased absorption of medications secondary to nasal discharge. These patients may experience a strong or obstructed response to intranasal medication. In the clinical evaluation, total serum testosterone concentrations were decreased by 21-24% in men with symptomatic allergic rhinitis, either treated with nasal decongestants or not treated. Treatment with intranasal testosterone should be delayed until symptoms are resolved in patients with nasal congestion, allergic rhinitis, or upper respiratory infection. If severe symptoms of rhinitis persist, alternative testosterone replacement therapy is recommended. The safety and effectiveness of the topical testosterone products Androgel, Axiron, Fortesta and Testim, as well as Striant oral tablets, Natesto intranasal gel and testosterone injection Aveed have not been established in neonates, infants, children and adolescents PregnancyThe testosterone contraindicates during pregnancy due to possible adverse effects in the fetus category Women with child potential who receive testosterone treatment should use appropriate contraceptive methods. Because testosterone is not used during pregnancy, there should be no particular reason for administering products to women during childbirth or obstetric delivery; safety and effectiveness in these environments have not been established. Breastfeeding The topical solution of testosterone, transdermal patches and gels are contraindicated in nursing women. It is recommended to avoid other testosterone formulations during breastfeeding as well. The distribution of testosterone to breast milk has not been determined; it is unclear whether the exposure would increase above the levels normally found in human milk. Significant exposure to this androgen through breastfeeding may have adverse androgenic effects on the baby and the medication may also interfere with the proper establishment of breastfeeding in the mother. Historically, testosterone/androgens have been used adjunctively for the suppression of breastfeeding. Alternative methods for breastfeeding are recommended for infants who receive testosterone therapy. Interactions Possible interactions include: certain diabetes medications; certain medicines that treat or avoid blood clots such as warfarin; oxyphenobutazone; propranolol; steroid medicines such as prednisone or cortisone. This list cannot describe all possible interactions. NOTE: Testosterone is a substrate for liver cytochrome P450 (CYP) 3A4 isoenzyme. Testosterone is also transported by and a P-glycoprotein transport inhibitor. Testosterone can increase the anticoagulant action of warfarin. Serious bleeding has been reported in some patients with this interaction with the medication. Although the mechanism is not clear, testosterone can reduce procoagulative factors. The reduction of the dose of warfarin may be necessary if testosterone therapy is coadministered. More frequent monitoring of RIN and protrombin time is recommended in patients taking such oral anticoagulants, especially in the initiation and termination of androgen therapy. It is not clear whether testosterone can increase the anticoagulant response to heparin therapy or whether testosterone alters the effect of other non-coumarin oral anticoagulants in a similar way. Based on the reports of cases with methyltetosterone and danazole, androgens can increase plasma concentrations of cyclosporine, leading to increased risk of nephrotoxicity. The administration of corticosteroids and testoterone may increase the risk of edema, especially in patients with underlying heart or liver disease. Corticosteroids with greater mineralocorticoid activity, such as fludrocortisone, can be more likely to cause edema. Manage these medicines in combination with caution. Goserelin and leuprolide inhibit steroids. Concomitant use of androgens with goserelin or leuprolide is relatively contraindicated and would defeat the purpose of goserelin or leuprolide therapy. Androgens can increase the risk of hepatotoxicity and therefore should be used with caution when administered concomitantly with other hepatotoxic drugs. Patients should be closely monitored for signs of liver damage, especially those with a history of liver disease. Androgens can be needed to help in the response to growth to human growth hormone, but excessive androgen doses in prepubescent men can accelerate epiphyseal maturity. Androgens are known to stimulate erythropoiesis. Despite the fact that the endogenous generation of erythropoietin is depressed in patients with chronic kidney failure, other tissues besides kidney can synthesize erythropoietin, although in small quantities. Concurrent androgen administration can increase the patient's response to alpha epoetin, reducing the amount needed to treat anemia. Because adverse reactions have been associated with an abrupt increase in blood viscosity, this combination of drugs should be avoided, if possible. A further assessment of this combination is necessary. The antiandrogenic effects of 5 alpha reductase inhibitors (i.e. dutasteride, finasterida) are antagonistic to the actions of androgens; it would be illogical for patients taking androgens to use these antiandrogen drugs. Drug interactions with Saw palmetto, Serenoa repens have not been specifically studied or reported. The palmetto extracts of saw seem to have antiandrogenic effects. The antiandrogenic effects of Saw's palm, Serenoa repens are expected to antagonize the actions of androgens; it would seem illogical for patients taking androgens to use this herbal supplement. Limited data suggest that testosterone concentrations increase during fluconazole administration. It seems that the fluconazole doses of 200 mg/day or more are more likely to produce this effect than the doses of 25—50 mg/day. The clinical meaning of this interaction is not clear at this time. Although data are not available, a similar reaction with voriconazole may occur. Both fluconazole and voriconazole are inhibitors of CYP3A4, the hepatic microsomal isoenzyme responsible for the metabolism of testosterone. Exogenously administered androgens (from latestosterone derivatives or anabolic steroids) have variable effects on blood glucose control in patients with diabetes mellitus. In general, low levels of testosterone are associated with insulin resistance. Furthermore, when hypogonadal men (with or without diabetes) are administered androgen exogenous, glucemic control generally improves as indicated by significant reductions in plasma glucose and HbA1c concentrations. In a study in men with diabetes, testosterone non-dedecenoate 120 mg PO/day for 3 months decreased HbA1c concentrations from a base of 10.4% to 8.6% (p In vitro, both genisteine and daidzein inhibit 5 isoenzima alpha-reductase II, which leads to a decrease in the conversion of testosterone to the subsequent potent andrDH The action is similar to that of finasteride, but it is believed to be less powerful. Theoretically, because soy isoflavones seem to inhibit type II 5-alpha-reductase, soy isoflavones can counteract androgen activity. Conivaptan is a powerful CYP3A4 inhibitor and can increase plasma concentrations of drugs that are metabolized mainly by CYP3A4. Testosterone is a substrate for CYP3A4 isoenzymes. The clinical meaning of this theoretical interaction is not known. Testosterone is a P-glycoprotein transport inhibitor. Ranolazine is a P-glycoprotein substrate, and P-glycoprotein inhibitors can increase the absorption of ranolazine. In addition, CYP3A inhibits froolazine and can increase plasma concentrations of drugs that are metabolized mainly by CYP3A4 as testosterone. Ambrisentan is a substrate for P-glycoprotein transport, an effluent pump of energy-dependent drugs. P-glicoprotein inhibition, by medications such as testosterone, may lead to a decrease in intestinal metabolism and an increase in the oral absorption of ambrisentan. If ambrisentan is administered jointly with a glycoprotein P inhibitor, patients should be monitored closely for adverse effects. Oxyphenbutazone co-administration and testosterone can lead to high concentrations of oxyphenbutazona. Supervising patients for adverse effects when they co-administrate these medications together. Testosterone cypionate has been shown to increase the cleaning of propranolol in a study. Monitoring patients taking testosterone and propranolol together to reduce the therapeutic efficacy of propranolol. The administration of dabigatran and testosterone can lead to increased concentrations of serum dabigatran and therefore to a higher risk of adverse effects. The administration of dabigatran and testosterone should be avoided in patients with severe kidney deficiency (CrCl 15-30 ml/min). Dabigatran is a P-gp substrate; testosterone is a P-gp inhibitor. P-gp inhibition and kidney impairment are the main independent factors that cause increased exposure to dabigatran. Concomitant use of testosterone, a P-gp inhibitor (P-gp) and afatinib, a P-gp substrate, can increase exposure of afatinib. If the use of both agents is necessary, consider reducing the dose of afatinib if the original dose is not tolerated. Concomitant use of intranasal testosterone (e.g., Natesto) and other intranasally administered drugs is not recommended; the potential for drug interaction among these agents is unknown. Eighteen men with seasonal allergic rhinitis were treated with intranasal and randomized testosterone to receive oxymetazoline (30 minutes before intranasal testosterone) or without treatment. Overall, total serum testosterone concentrations decreased by 21-24% in men with symptomatic allergic rhinitis, due to the underlying condition. An average decrease was observed in AUC and Cmax (2.6% and 3.6% respectively) for total testosterone in men with symptomatic seasonal rhinitis when treated with oxymetazoline compared to nontreated patients. Concomitant use of oxymetazoline does not affect the absorption of testosterone. This list may not include all possible interactions. Give your health care provider a list of all medicines, herbs, prescription medications, or dietary supplements you use. Also tell them if you smoke, drink alcohol or use illegal drugs. Some elements may interact with your medication. Adverse Reactions/ Side Effects Male patients may experience feminization during protracted therapy with testosterone, which is believed to result from inhibiting the secretion of gonadotropin and converting androgens to estrogens. These effects are more pronounced in male patients with concurrent liver disease and include mastalgia and gynecomastia. In clinical evaluation of testosterone gel, gynecomastia (Testim: 1%; Androgel: 1–3%) and mastalgia (Androgel: 1–3%) were reported. Mastalgia and increased blood testosterone were reported in less than 1% of patients who took Axiron. The feminizing effects of testosterone are generally reversible. During the exogenous administration of androgens, the release of endogenous testosterone is inhibited by inhibiting feedback from pituitary luteinizing hormone (HL). In large doses of exogenous androgens, the inhibition of spermatogenesis can occur through the inhibition of the feedback of the pituitary follicle stimulating hormone (FSH). Similar to other testosterone therapies, decreased testosterone and oligospermia have been reported during the subsequent monitoring of the approval of the topical gel of testosterone. Testosterone therapy may result in decreased libido or increased libido. In the clinical evaluation of testosterone gel (Androgel), the decrease in libido was reported in 1–3% of patients. Priapism and excessive sexual stimulation, most common in geriatric men, are generally the effect of excessive doses of testosterone. In 205 patients who received testosterone gel (Testim 50 or 100 mg daily), the spontaneous erection of the penis (1%) was reported. During the post approval experience with testosterone (Fortesta), priapism and impotence (thermal dysfunction) were reported. Prostatic hypertrophy can develop during prostate therapy with testosterone and these events are more likely to occur in older male patients. In 205 patients who received testosterone gel (Testim 50 or 100 mg daily), benign prostatic hyperplasia, BPH was reported in 1% of patients. Clinical trials for testosterone patch (Androdermo) include reports of unspecified prostate abnormalities in 5% of patients. Prostate neoplasia was reported in less than 1% of patients who took Axiron. In addition, increases in serum PSA concentrations have been reported in clinical trials for the topical testosterone solution (Axiron: 1-4%), topical gel (Fortesta: 1.3%), and intranasal gel (Natest: 5.1-5.8%). In a 180-day study, phase 3 of the testosterone gel (Androgel), prostate disorder (3-5%) including expanded prostate, BPH and elevated PSA were reported; testis disorder (1.9-3%) including left varicocele and light testicular sensitivity. In 162 hypogonadal men who received testosterone gel (Androgel) during a 3-year extension trial, increases were observed in serum PSA concentrations (defined as reference concentrations √= 2x or any single absolute value √= 6 ng/ml) in approximately 18% of patients (n = 29). Most of these increases were observed in the first year of therapy (23/29 or 79%). Four patients had a single value 6 ng/ml: 2 of these patients had detected prostate cancer in the biopsy. In the same study, symptoms of prostate and expanded urinary tracts were also reported, including nocturia, urinary hesitancy, urinary incontinence, urinary retention, urinary urgency and weak urinary flow. Finally, a patient reported prostate disorder that required a transuretral resection of the prostate (TURP) considered possibly related to the treatment by researchers. Dysuria and hematuria have also been reported during postmarketing surveillance of testosterone therapy. Hematuria (When the androgens are given to the females, virilization, manifested by acne, the growth of facial hair or an unwanted excess of body hair (hirsutism), enlarged clitoris, reduced breast size, and the deepening of the voice, may occur. If the treatment of testosterone is suspended when these symptoms first appear, they are usually subsided. Post-approval reported dermatological reactions or topical testosterone products are associated with skin reactions from the application site. In clinical studies with testosterone patch (Androdermo), mild to moderate transient erythema was observed at the application site in most patients at some time during treatment. The overall incidence of any type of site reactions was 28% (10 subject with 13 adverse reactions). The adverse events of the reported application site include: pruritus (17-37%), ampoules reaction as a low system burn (12%), erythema ( 1%) was reported in a clinical evaluation of the testosterone solution (Axiron). Alopecia that resembles male baldness has also occurred in patients who receive long-term therapy or excessive doses of testosterone. Dermatological reactions reported post-approval or in The oral mucoadhesive system of testosterone can cause dental pain, such as gum irritation or mouth (9.2%), a bitter taste in the mouth (disgeusia, 4.1%), gum pain (3.1%), gum tenderness (3.1%), gum edema (2%), or taste perversion (2%). Most of the rubber-related adverse events were transient; rubber irritation was usually resolved in 1-8 days and the rubber tender was resolved in 1-14 days. The following adverse events occurred in 1 patient during clinical trials: mucusal agitation, gingivitis, blisters, nose edema, lip itching and tooth pain. In clinical trials, 4.1% of patients suspended treatment due to gum or side events related to the mouth. A study conducted gum tests to evaluate gingivitis, gum edema, oral lesions, oral ulceration or leucooplakia without new or worse cases of any of these reported anomalies. In two long-term extension trials, the following adverse events occurred in 1 patient each: oral inflammation, xerostomia, gum redness, stomatitis, bitter taste/perversion of flavor (dysgeusia), and tooth pain. Dysgeusia (reported as a taste disorder) was reported in 1% of patients who received testosterone gel (Testim) and possibly tried, probably, or definitely related to the study medication. However, dysgeusia has not been observed as a side effect with other topical or injectable testosterone products and topical and systemic testosterone is not recognized as a common cause of taste alteration. Early exposure to pharmaceutical doses of testosterone or other androgens in pre-puberal men can induce virilism that can be a disadvantage because it is accompanied by premature epiphyseal closure. Once the epiphytes are closed, growth is over. Monitoring of skeletal ripening should be performed at intervals of approximately 6 months. Once the epiphytes are closed, growth is over. Even after the interruption of the treatment of testosterone, epiphyseal closure can be improved for several months. Androgen therapy has been associated with sodium retention, chloride, water, potassium and inorganic phosphates. Peripheral edema may occur as a result of increased fluid retention (in association with sodium chloride) and may manifest by weight gain. These effects can be more prominent before in androgen therapy. If normal testosterone doses are used in the treatment of hypogonadism, only a moderate amount of fluid retention is produced. In the treatment of patients with kidney failure or congestive heart failure, fluid retention is of greater importance. Animal models suggest the ability of testosterone to induce blood pressure increases and alter naturesis thus affecting vasoconstriction and stimulation of the renin-angiotensin-aldosterone system. Therefore, androgens can affect blood pressure; however, the current role of testosterone in blood pressure regulation is not well understood. Hypertension has been reported during the clinical evaluation, as well as monitoring after the approval of testosterone therapy. In clinical trials, 2.1%—3% of patients who received testosterone gel (Androgel) reported hypertension. Hypertension (1%) as well as decreased diastolic pressure (1%) were reported in testosterone gel (Testim). Hypertension (conference1%) was reported in patients with testosterone topical solution (Axiron). In addition to affecting blood pressure, androgens may affect the prevalence of cardiovascular disease. The possible association between the use of testosterone and the increased risk of serious cardiovascular events, regardless of pre-existing heart disease, is currently under investigation. An observational study in the U.S. Veterans' Health System included adult male patients of an average age of 60 years. Patients (n = 8709) suffer coronary angiography with a low serum testosterone concentration of = 65 years old) adult males (n = 55.593). The incidence rate of MI that occurred within 90 days after the initial testosterone prescription was compared to the rate of MI incidence that occurred in the year prior to the first prescription. Among older men, an increase of 2 times in the risk of MI was observed within the 90-day window; among younger men with pre-existing history of heart disease, an increase of 2 to 3 times of risk of MI was observed. However, there was no greater risk in younger men without a history of heart disease. In light of these findings, the FDA announced in early 2014 a review of the possible link between testosterone therapy and serious cardiovascular events. FDA has not concluded that the treatment of testosterone approved by FDA increases the risk of stroke, MI or death. However, health professionals are urged to consider carefully whether treatment benefits are likely to exceed potential risks. The FDA will communicate its final conclusions and recommendations when the evaluation is complete. Hepatic dysfunction can occur from the use of certain androgens; therefore, periodic monitoring of liver function is recommended. Using as prescribed, elevated liver enzymes are more likely to occur than excess jaundice or other liver dysfunction, which are rare with the use of testosterone in general. The adverse liver effects are more prone to the administration of 17 alpha-alkylagens (e.g. methyltestosterone) or to the abuse of such androgenic hormones by athletes, where the results of abuse in liver changes consisting of hepatic fat disease (esteatosis) in an estimated 2.4% of individuals, even in the absence of other risk factors for fatty liver. Testosterone should be suspended if colstatic jaundice or hepatitis or other adverse liver dysfunction occurs. Hepatis of peliosis and hepatic neoplasms rarely occur, but when they do, they are potentially fatal. Head pain has been reported in several testosterone therapy trials; headache incidence rates range from 1 to 6%, regardless of formulation. Some incidences of mood disorders, including emotional lability ( 1%), anger ( Rabbit 1%), astenia (Testosterone therapy has induced osteolysis and can exacerbate hypercalcemia. Androgen-induced hypercalcemia occurs especially in immobile patients and those with metastatic breast carcinoma. The skeletal adverse reactions reported during the monitoring of post-testosterone undecanate approval include osteopenia and osteoporosis. Testosterone can cause undesirable changes in serum lipid profiles, including hypercholesterolemia or hypertriglyceremia. Periodic monitoring of lipid profiles can be desirable during treatment. Observational studies in post-menopausal women, bodybuilders and weight lifters using anabolic steroids have revealed "pro-aterogenic" changes in lipid profiles, including decreases in HDL concentrations and increases in LDL concentrations. Synthetic androgens can lead to a greater reduction in HDL-C:LDL-C ratio than testosterone. Although the implications of androgen-induced hypercholesterolemia are unclear, caution must be exercised, especially in patients predisposed to dyslipidemia or atherosclerosis. If lipid changes are significant, a testosterone or lipid dose adjustment may be needed to reduce medications or suspend the treatment of testosterone; identify therapy. Testosterone has an exhilarating effect on the formation of erythropoietin. The increase in erytropoiesis, especially in women, can lead to erythrocytosis, secondary polycytemia, and its complications, including: dizziness, migraines, fatigue (fatigue), unusual bleeding, blushing or redness of the skin. Patients who receive high doses of testosterone are at risk of polycytemia. In the clinical evaluation of the testosterone solution (Axiron), increases in the count of red blood cells ( 1%) were reported. In testosterone gel (Testim), patients who received a dose of 100 mg had clinically significant increases in hematocrite (2.8%) and hemoglobin (2.3%). Similarly, 2.1 per cent of patients treated with testosterone gel (Androgel 1.62%) reported increased hematocrite or hemoglobin. In intranasal testosterone gel analysis, 4 out of 306 patients exposed developed a hematocrite level of 55% (baseline: 48-51%; not exceeding 58%). Therefore, periodic determinations of hemoglobin and hematocrit should be considered in patients receiving long-term testosterone therapy. In general, testosterone therapy has been associated with the suppression of coagulation factors II, V, VII and X and bleeding in patients with concomitant anticoagulant therapy. GI bleeding was reported in 2% of patients who received testosterone (Androderm) patch treatment during the clinical evaluation. Hemartrosis (Intramuscular administration of anabolic steroids can cause inflammation, erythema, urticaria, post-injection pain, induration and furunculosis. Inflammation and pain may be possible in the pellet insertion site of testosterone implant. Testosterone pellets can also leave the insertion site, which is usually secondary to the superficial or aseptic implantation. Patients should be observed by any reaction sign at the injection site. Few cases of anaphylactoid reactions have been reported in association with oral and injectable testosterone therapy. The administration of the undecanate of testosterone has been associated with cases of pulmonary embolism, specifically severe reactions of pulmonary oil microembolism (POME) as well as anaphylactoid reactions. Reported cases of POME reactions occurred during or immediately after an intramuscular injection of 1000 mg of undecanate testosterone. Symptoms included: coughing, coughing, dyspnea, hyperhydrosis, tightening of the throat (high bronchospasm), chest pain, dizziness and syncope. Most cases lasted a few minutes and were resolved with support measures; however, some lasted for several hours and some of them required emergency care and/or hospitalization. In addition to POME reactions, episodes of anaphylaxis have been reported, including potentially fatal reactions, following intramuscular injection of a testosterone deanate. In total, 9 POME events in 8 patients and 2 anaphylaxis events between 3556 patients treated with a testosterone deanate were reported in 18 clinical trials; cases of PME and post-approval anaphylaxia were also reported. Cases have occurred after initial injection, as well as during subsequent injections during the normal course of treatment. After each administration, monitor the patient for 30 minutes and provide appropriate medical treatment in case of severe reactions of POME or anaphylactoid. Due to the risk of severe reactions of POME and anaphylaxia, undecanate testosterone (Aveed) is only available through a restricted program called REMS Aveed Program. Clinicians who wish to prescribe Aveed must be certified with the REMS Program to order or dispens the product. Medical care settings should also be certified with the REMS Program and should have the resources to provide emergency medical treatment in cases of severe PME and anaphylaxia. More information is available at www. AveedREMS.com or call 1-855-755-0494. Transient respiratory reactions, including cough impulse, cough settings and respiratory distress immediately after intramuscular injection of testosterone enantalate have been reported during post-marketing surveillance. Be careful to ensure the slow and deep injection of gluteal muscle from the preparations for testosterone. Nasopharyngitis or pharyngitis ( 1%) was reported in patients who received topical testosterone (Axiron). Treatment of hypogonadal men with testosterone may increase the risk of sleep apnea, especially in patients with risk factors for sleep apnea, such as obesity or chronic lung disease. In the clinical evaluation of intranasal testosterone gel, the following adverse nasal reactions were reported among the most common adverse events: nasopharyngitis (3.8-8.7%), rhinorene (3.8-7.8%), parose (5.8%), epistaxis (3.8-6.5%), nasal irritation or discomfort (3.8-5.9%), nasal spabullition (3.8-5.8%). Although most nasal complaints were mild or moderate, long-term nasal safety data are limited. Advise patients to report any nasal symptoms that affect; if they are present, determine the need for a subsequent evaluation or continued treatment. Other reported adverse respiratory reactions include bronchitis (3.8-4.3%), upper respiratory tract infection (3.8-4.3%) and sinusitis (3.8%). Call your healthcare provider immediately if you are experiencing any signs of an allergic reaction: skin rash, itching, or hive, swelling of the face, lips or tongue, blue skin dye, chest stiffness, pain, shortness of breath, wheezing, dizziness, red, swollen painful area in the leg. StorageRelated addictionsProductsCompanyOrderingGet in Touch Do you have any questions or comments about our website, our products or any of our services? Follow us© 2020 Empower Pharmacy
8 Undesired side effects of testosterone or Gel cream About testosterone and testosterone topic Testosterone Testosterone is a typically male hormone that occurs mainly in testicles. If you are a man, help your body develop sexual organs, sperm, and sexual unity. The hormone also helps to maintain male features such as muscle strength and mass, facial and body hair, and a deep voice. Testosterone levels tend to reach early adulthood and. Topic testosterone is a prescription medication that applies to your skin. It is used to treat , a condition that prevents your body from doing enough testosterone. Topical testosterones approved in gel form. However, some men prefer composite creams of testosterone (where a pharmacy mixes testosterone with a creamy base), because they are easier to use and less likely to be transferred by touch. Otherwise, the effects of gels vs. creams are not very different. Although topical testosterone can be useful for men with hypogonadism, it can also cause topical and unexpected hormonal side effects. The most common side effects of topical testosterone are skin reactions. Because it applies to topical testosterone directly to your skin, you can develop a reaction on the application site. Symptoms may include: Be sure to always apply the medication on the skin clean and without breaking. Carefully follow the package application instructions and report any skin reaction to your doctor. Topic testosterone may also affect your urinary tract. Some men need to urinate more than usual, even during the night. You may feel an urgent need to urinate, even when your bladder is not full. Other symptoms include urination and blood difficulty in the urine. If you are using topical testosterone and have urinary problems, talk to your doctor. Hypogonadism can cause gynecomastia (enlarged) in men. It's rare, but the use of topical testosterone can bring unwanted changes to the breasts. This is because your body changes a testosterone in a form of hormonal estrogen, which may result in your body forming more breast tissue. Breast changes may include: If you are concerned about changes in your breasts while using topical testosterone, see your doctor immediately. Tapic testosterone can let you sit a little out of class. Symptoms are not common, but they may include feeling dizzy, clear or weak head. Sometimes the use of topical testosterone can cause hot flashes or hitting sounds in the ears. These symptoms may be leakage and may disappear on their own. If you are still a problem, talk to your doctor. Most men tolerate the treatment of testosterone quite well, but a small number develops emotional side effects of hormonal changes. These may include: Although emotional side effects are rare, they may be serious. Be sure to discuss any symptoms with your doctor. Testosterone plays a great role in a man's sexual unity. But in rare cases, topical testosterone can negatively affect sexuality. It can cause problems like:Call your doctor if you have any of these symptoms and get upset. Topic testosterone can cause side effects on women and children who come into contact with her on their skin or clothing. Children can develop aggressive behavior, enlarged genitals and pubic hair. Women can develop unwanted growth of the hair or acne. The transfer of testosterone is especially dangerous for pregnant women because it can cause birth defects. Women and children exposed to testosterone products should call their doctor immediately. To prevent these problems, do not allow skin-to-skin contact in the area treated with other people. Keep the treated area covered or wash well before letting others touch you. Also, do not allow others to touch any bed linen and clothing that may have absorbed the testosterone from your skin. The FDA has issued a higher risk of cardiovascular events among men using testosterone products. Be sure to check with your healthcare provider about this potential problem. Topic testosterone is a powerful prescription medication that should only be used under your doctor's supervision. It can cause side effects other than those we mentioned, so talk to your doctor if you have any questions. Some side effects can be clarified by themselves, but some may require medical care. Be sure to report any side effects to your doctor. Also be sure to tell your doctor if you have other health conditions, including: Tell them about other prescription and over-the-counter medications and supplements you are taking and ask about any possible interaction with medicines. Last medical review on October 9, 2019Read this following

Testosterone cream cycle for sale: Testosterone Gel Hair Routine | #Glamidays Day 19 | @ashleidominique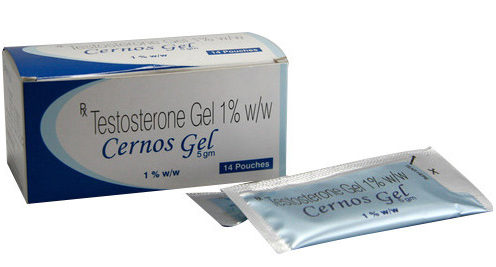
Buy Cernos Gel. Generic Androgel. India Prices, Shipping to US, AU, UK
Buy AndroGel transdermal gel online (chemresearchshop.com) for sale Philippines - Find New and Used Buy AndroGel transdermal gel online (chemresearchshop.com) for sale on BuyandSellPH
Testosterone Gel (AndroGel) | Men's Health Singapore
The Official AndroGel (testosterone gel) 1.62% CIII Website
Testosterone Cream for Sale | Buy Testosterone Cream Online
Testosterone Cream for Sale | Buy Testosterone Cream Online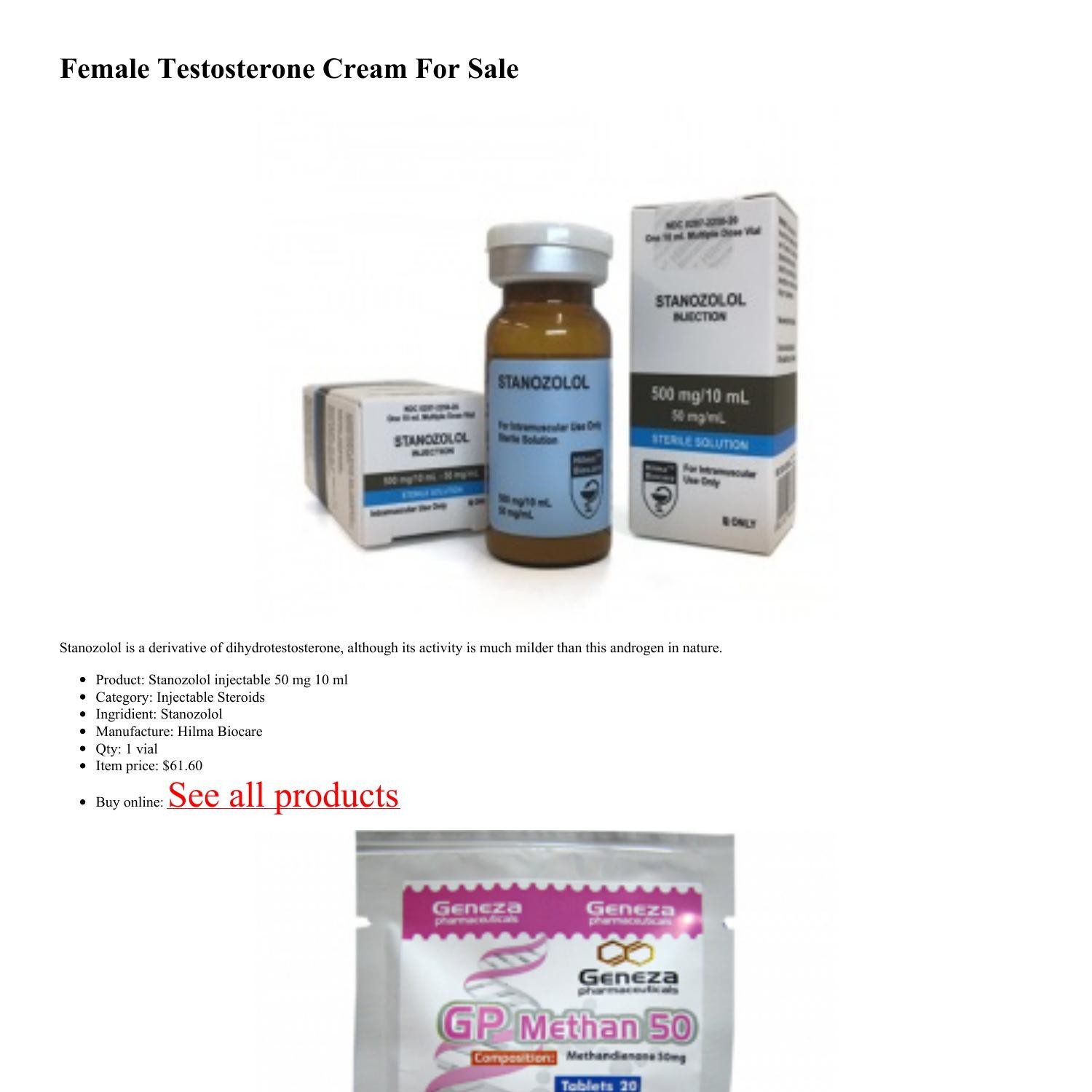
Female Testosterone Cream For Sale.pdf | DocDroid
Buy Libido Edge Labs - TestaEdge Cream for Women - 4 oz. formerly Testosterone Cream Formula for Women at LuckyVitamin.com
A Push to Sell Testosterone Gels Troubles Doctors - The New York Times
Buy Testim Gel | Testosterone Gel Treatments for Sale Online UK
Steroids canada review - Durabolin
Female Testosterone Cream For Sale.pdf | DocDroid
11 Libido Booster ideas | libido, dhea, dhea supplement
Androgel | Lowtiyel for Sale | Testosterone Enhancement in Mexico
HRT and Me: Using Testim Testosterone Gel as a Woman - Write Health
Buy Tostran Testosterone Gel Online
11 Libido Booster ideas | libido, dhea, dhea supplement
ANDROGEL Buy For Less. Genuine Besins (France)
A Push to Sell Testosterone Gels Troubles Doctors - The New York Times
Testosterone Rx - Buy Online – No Prescription Required
Buy Androgel — Testosterone gel buy online us
Buy Cernos Gel. Generic Androgel. India Prices, Shipping to US, AU, UK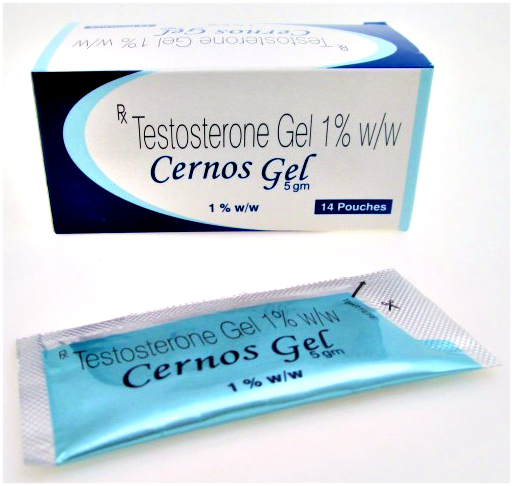
Buy Androgel Online Generic Testosterone Gel 1% | India Prices
Testosterone Cream UNLEASH YOUR WOLF Tongkat Ali, Maca Extract Libido Gel - 3B for sale online
Buy Testosterone Gel Testogel Online UK | Prescription Doctor
Testosterone Gel For Sale
Scrotal Testosterone Cream - Can it Increase Libido
Testosterone replacement - Dr Grant Fourie
Testosterone Cream, 30mL | Defy Medical
Testosterone cream cycle for sale: Testosterone Gel Hair Routine | #Glamidays Day 19 | @ashleidominique
Topical Testosterone: 8 Unwanted Side Effects
Testosterone Gel (AndroGel) | Men's Health Singapore
Buy Tostran Gel from £37.49 each - UK Meds™ Online Pharmacy
Testosterone Gel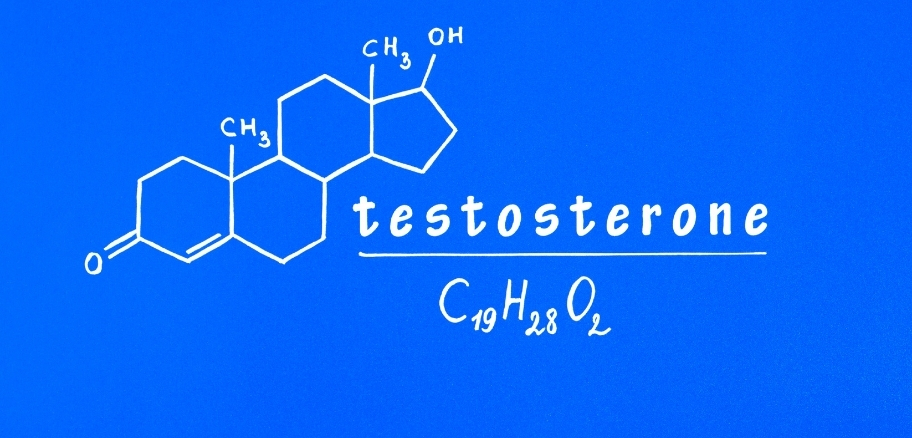
Testosterone Cream Vs Testosterone Injections | Victory Mens Health
How effective are testosterone pellets: Side effects and benefits
How To Increase Testosterone Naturally: 32 Tips For Men And Women
Testosterone Cream for Sale Online for Men & Women – Apply Over the Counter.
Buy Low Testosterone Gel Treatment Online - UK Pharmacy
 TESTAEDGE Cream for Men Testosterone Booster Anti-aging Ed Libido 4 Oz for sale online | eBay
TESTAEDGE Cream for Men Testosterone Booster Anti-aging Ed Libido 4 Oz for sale online | eBay

































Posting Komentar untuk "testosterone cream for sale"